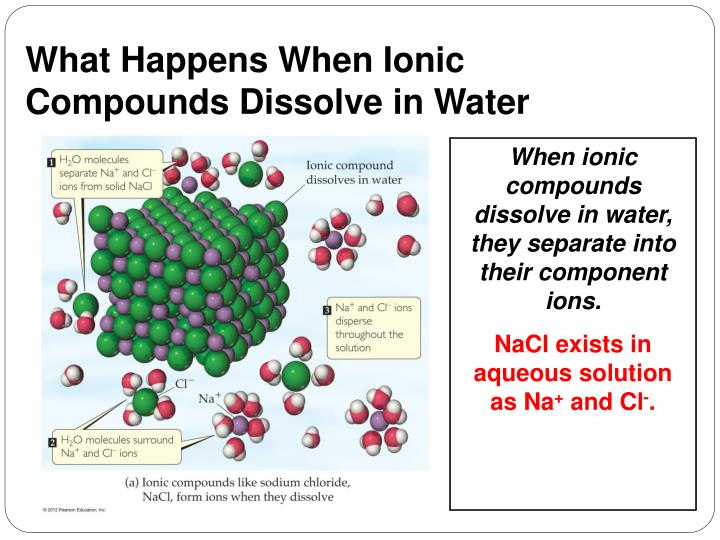
Why Do Ionic Compounds Dissolve In Water Simple . — when an ionic compound is dissolved in water a special interaction. dissolution of ionic compounds occurs in three steps: — when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly. But when a salt dissolves, at least 4 imf bonds are formed. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. — an individual imf bond is weaker than an ionic bond. — learn why ionic compounds dissolve in water and how they become electrolytes. — when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly. Separation of solvent molecules is endothermic as energy is absorbed to overcome intermolecular. Find out the factors that affect solubility and the examples of ionic. when ionic compounds dissolve in water, the negative end of the water molecule is attracted to the cation.
from www.slideserve.com
Find out the factors that affect solubility and the examples of ionic. Separation of solvent molecules is endothermic as energy is absorbed to overcome intermolecular. dissolution of ionic compounds occurs in three steps: — when an ionic compound is dissolved in water a special interaction. — when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly. — an individual imf bond is weaker than an ionic bond. when ionic compounds dissolve in water, the negative end of the water molecule is attracted to the cation. — learn why ionic compounds dissolve in water and how they become electrolytes. But when a salt dissolves, at least 4 imf bonds are formed. — when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly.
PPT UNIT 5 PowerPoint Presentation ID2276550
Why Do Ionic Compounds Dissolve In Water Simple — when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly. — learn why ionic compounds dissolve in water and how they become electrolytes. — when an ionic compound is dissolved in water a special interaction. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. when ionic compounds dissolve in water, the negative end of the water molecule is attracted to the cation. Find out the factors that affect solubility and the examples of ionic. Separation of solvent molecules is endothermic as energy is absorbed to overcome intermolecular. — when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly. But when a salt dissolves, at least 4 imf bonds are formed. dissolution of ionic compounds occurs in three steps: — an individual imf bond is weaker than an ionic bond. — when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly.
From www.slideserve.com
PPT Solutions and Mixtures PowerPoint Presentation, free download ID9165094 Why Do Ionic Compounds Dissolve In Water Simple Separation of solvent molecules is endothermic as energy is absorbed to overcome intermolecular. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. dissolution of ionic compounds occurs in three steps: — when an ionic compound is dissolved in water a. Why Do Ionic Compounds Dissolve In Water Simple.
From www.chegg.com
Solved Why do solid ionic compounds dissolve in water? A. Why Do Ionic Compounds Dissolve In Water Simple But when a salt dissolves, at least 4 imf bonds are formed. — when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly. — when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly. dissolution of ionic compounds occurs in three steps: ionic compounds dissolve in. Why Do Ionic Compounds Dissolve In Water Simple.
From shareeducatonideas.com
What Is An Ionic Compound? Formula and Defination Why Do Ionic Compounds Dissolve In Water Simple — when an ionic compound is dissolved in water a special interaction. But when a salt dissolves, at least 4 imf bonds are formed. when ionic compounds dissolve in water, the negative end of the water molecule is attracted to the cation. — when ionic compounds dissolve in water, the ions in the solid separate and disperse. Why Do Ionic Compounds Dissolve In Water Simple.
From www.slideserve.com
PPT Solution Chemistry PowerPoint Presentation, free download ID2871797 Why Do Ionic Compounds Dissolve In Water Simple when ionic compounds dissolve in water, the negative end of the water molecule is attracted to the cation. Separation of solvent molecules is endothermic as energy is absorbed to overcome intermolecular. — when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly. — when an ionic compound is dissolved in water a. Why Do Ionic Compounds Dissolve In Water Simple.
From www.youtube.com
Why Ionic Compounds Dissolve in Water ? Chemistry Facts Prateek Agarwal YouTubeshorts Why Do Ionic Compounds Dissolve In Water Simple — an individual imf bond is weaker than an ionic bond. Find out the factors that affect solubility and the examples of ionic. — when an ionic compound is dissolved in water a special interaction. dissolution of ionic compounds occurs in three steps: when ionic compounds dissolve in water, the negative end of the water molecule. Why Do Ionic Compounds Dissolve In Water Simple.
From www.youtube.com
Predicting solubility of ionic compounds in water YouTube Why Do Ionic Compounds Dissolve In Water Simple dissolution of ionic compounds occurs in three steps: — when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly. Find out the factors that affect solubility and the examples of ionic. — when an ionic compound is dissolved in water a special interaction. — when ionic compounds dissolve in water, the. Why Do Ionic Compounds Dissolve In Water Simple.
From www.youtube.com
Why do alcohol (a covalent compound) dissolves in water (ionic)? YouTube Why Do Ionic Compounds Dissolve In Water Simple Find out the factors that affect solubility and the examples of ionic. — an individual imf bond is weaker than an ionic bond. But when a salt dissolves, at least 4 imf bonds are formed. — learn why ionic compounds dissolve in water and how they become electrolytes. ionic compounds dissolve in water if the energy given. Why Do Ionic Compounds Dissolve In Water Simple.
From www.slideserve.com
PPT Dissolving of an Ionic Compound PowerPoint Presentation, free download ID3851009 Why Do Ionic Compounds Dissolve In Water Simple — when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly. — when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly. Separation of solvent molecules is endothermic as energy is absorbed to overcome intermolecular. when ionic compounds dissolve in water, the negative end of the water. Why Do Ionic Compounds Dissolve In Water Simple.
From 2012books.lardbucket.org
The Dissolution Process Why Do Ionic Compounds Dissolve In Water Simple ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. — an individual imf bond is weaker than an ionic bond. — when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly. dissolution of ionic. Why Do Ionic Compounds Dissolve In Water Simple.
From www.slideserve.com
PPT The Chemical & Physical Basis of Life PowerPoint Presentation ID4223253 Why Do Ionic Compounds Dissolve In Water Simple — an individual imf bond is weaker than an ionic bond. Find out the factors that affect solubility and the examples of ionic. But when a salt dissolves, at least 4 imf bonds are formed. — when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly. Separation of solvent molecules is endothermic as. Why Do Ionic Compounds Dissolve In Water Simple.
From www.slideserve.com
PPT Chapter 4 Aqueous Reactions and Solution Stoichiometry PowerPoint Presentation ID6291419 Why Do Ionic Compounds Dissolve In Water Simple — when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly. — when an ionic compound is dissolved in water a special interaction. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. — when. Why Do Ionic Compounds Dissolve In Water Simple.
From www.slideserve.com
PPT Ionic Compounds and Metals PowerPoint Presentation, free download ID6037545 Why Do Ionic Compounds Dissolve In Water Simple — learn why ionic compounds dissolve in water and how they become electrolytes. — an individual imf bond is weaker than an ionic bond. when ionic compounds dissolve in water, the negative end of the water molecule is attracted to the cation. — when an ionic compound is dissolved in water a special interaction. Find out. Why Do Ionic Compounds Dissolve In Water Simple.
From www.slideshare.net
Properties of Compounds Ionic, Covalent and Metallic Why Do Ionic Compounds Dissolve In Water Simple — learn why ionic compounds dissolve in water and how they become electrolytes. Find out the factors that affect solubility and the examples of ionic. Separation of solvent molecules is endothermic as energy is absorbed to overcome intermolecular. — when an ionic compound is dissolved in water a special interaction. ionic compounds dissolve in water if the. Why Do Ionic Compounds Dissolve In Water Simple.
From www.slideserve.com
PPT Solutions & Solubility PowerPoint Presentation, free download ID7002512 Why Do Ionic Compounds Dissolve In Water Simple — an individual imf bond is weaker than an ionic bond. Separation of solvent molecules is endothermic as energy is absorbed to overcome intermolecular. Find out the factors that affect solubility and the examples of ionic. But when a salt dissolves, at least 4 imf bonds are formed. when ionic compounds dissolve in water, the negative end of. Why Do Ionic Compounds Dissolve In Water Simple.
From www.slideserve.com
PPT UNIT 5 PowerPoint Presentation ID2276550 Why Do Ionic Compounds Dissolve In Water Simple But when a salt dissolves, at least 4 imf bonds are formed. — an individual imf bond is weaker than an ionic bond. dissolution of ionic compounds occurs in three steps: when ionic compounds dissolve in water, the negative end of the water molecule is attracted to the cation. — when ionic compounds dissolve in water,. Why Do Ionic Compounds Dissolve In Water Simple.
From www.slideserve.com
PPT The Nature of Aqueous Solutions and Molarity and Solution Stoichiometry PowerPoint Why Do Ionic Compounds Dissolve In Water Simple dissolution of ionic compounds occurs in three steps: Find out the factors that affect solubility and the examples of ionic. Separation of solvent molecules is endothermic as energy is absorbed to overcome intermolecular. — when an ionic compound is dissolved in water a special interaction. — an individual imf bond is weaker than an ionic bond. . Why Do Ionic Compounds Dissolve In Water Simple.
From www.visionlearning.com
Solutions, Solubility, and Colligative Properties Chemistry Visionlearning Why Do Ionic Compounds Dissolve In Water Simple Separation of solvent molecules is endothermic as energy is absorbed to overcome intermolecular. — when an ionic compound is dissolved in water a special interaction. — learn why ionic compounds dissolve in water and how they become electrolytes. — when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly. dissolution of. Why Do Ionic Compounds Dissolve In Water Simple.
From www.slideserve.com
PPT Chapter 2 Water PowerPoint Presentation, free download ID262198 Why Do Ionic Compounds Dissolve In Water Simple — when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly. ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic. But when a salt dissolves, at least 4 imf bonds are formed. — an individual. Why Do Ionic Compounds Dissolve In Water Simple.